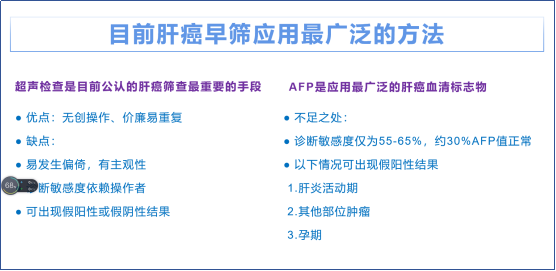
导读 肝内胆管细胞癌(ICC)是一种高度侵袭性的胆道恶性肿瘤,发病率呈逐年上升趋势,亚洲人群发病率明显高于欧美人群,临床上越来越受到重视。 根治性手术切除是目前可能治愈ICC的唯一方式,但仅30%-40%的患者存在手术切除的机会1-3。有效降低疾病分期、提高手术切除率是改善ICC病人生存率的重要途径,R0切除已被证明是提高生存率的关键因素4。因此,实现ICC缩瘤降期,扩大可手术切除患者的比例,实现患者的根治性手术切除,改善患者预后成为临床关注的重点方向。 本期与大家分享钇90选择性内放射治疗(SIRT)用于ICC新辅助降期缩瘤实现手术根治性切除的优势,为改善患者预后提供精准强效的“核武器”。 新辅助降期缩瘤,R0切除更获益 新辅助治疗是在主要治疗(通常是外科手术)之前给予肿瘤全身或者局部治疗,可实现肿瘤原发病灶的降期,控制淋巴结转移,提高R0切除率,从而降低复发风险、优化抗癌治疗,提高ICC病人的术后生存率5-6。 与大多数实体器官肿瘤一样,获得R0切除是一个重要的肿瘤学目标,也是外科医生可能控制的少数指标之一。R0切除定义为:完整切除术前和术中可发现的全部肿瘤病灶,且经组织病理学检查证实肿瘤切缘为阴性,肿瘤无肝外远处转移和大血管侵犯7。 在一项大型荟萃分析中,新辅助降期后接受切除的患者中位生存期显著长于未接受切除术的患者(29个月 vs 12个月)。Yeh等人报道,在224名患者的较大样本的研究中,R0切除患者的中位生存期为26.1个月,1年、3年和5年OS分别为78.5%、43.3%和28.6%,而R1切除患者的中位生存期为11.4个月,R2切除的结果更差,中位生存期为5.8个月8。 钇90微球助力新辅助手术根治性切除 目前还没有前瞻性随机试验评价新辅助化疗在ICC中的获益,一些病例报告、回顾性数据表明约有8~37%的患者在接受新辅助化疗后实现手术切除,R0切除率为31%8-11,经动脉化疗栓塞(TACE)的手术转化率为4~33%12-15,肝动脉灌注(HAI)的手术转化率为9~27%,R0切除率为4.7%16-17,而SIRT的手术切除率可达到52%~100%,R0切除率达到73%左右18-23,明显优于其他治疗方法。 在临床疗效长期获益方面,有研究显示在所有涉及到的新辅助疗法中,钇90微球的临床获益率最高,临床获益率(CR+PR+SD)可达到92%18,一项单中心队列研究纳入了136名不可切除ICC并接受钇90微球的治疗,研究结果显示中位复发时间为26.3个月,中位总生存时间为39.9个月22。 以上这些研究结果表明钇90微球提供了良好的局部区域控制,SIRT的加入可为ICC患者新辅助治疗带来降期效果,未来通过联合系统治疗可实现双赢,是未来临床试验和临床应用的方向。 参考文献 1. Hogdall D, O'Rourke CJ, Taranta A, Oliveira DV, Andersen JB. Molecular pathogenesis and current therapy in intrahepatic cholangiocarcinoma. Dig Dis. 2016;34(4):440–451. 2. Ellis MC, Cassera MA, Vetto JT, Orloff SL, Hansen PD, Billingsley KG. Surgical treatment of intrahepatic cholangiocarcinoma: outcomes and predictive factors. Hpb. 2011;13(1):59–63. 3. Hong K, Geschwind J-FH. Locoregional intra-arterial therapies for unresectable intrahepatic cholangiocarcinoma.SeminOncol. 2010;37(2):110–117. 4. 林志文, 刘红枝, 曾永毅, 等. 肝内胆管癌新辅助治疗的热点与进展[J]. 临床肝胆病杂志, 2023, 39(9): 2031-2038. 5. 张艳桥,郑桐森.肝内胆管癌新辅助治疗共识与争议[J]. 中国实用外科杂志,2020,40(6):688-691. 6. Medin CR, Maithel SK. Neoadjuvant therapy trials in biliary tract malignancies[J]. J Surg Oncol,2022, 125(1):84-88. 7. 原发性肝癌诊疗指南之肝内胆管癌诊疗中国专家共识(2022版). 8. Akateh C, Ejaz AM, Pawlik TM, Cloyd JM. Neoadjuvant treatment strategies for intrahepatic cholangiocarcinoma. World J Hepatol 2020; 12(10): 693-708. 9. Le Roy B. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Br J Surg. 2018 Jun;105(7):839-847. 10. Kato A. Downsizing Chemotherapy for Initially Unresectable Locally Advanced Biliary Tract Cancer Patients Treated with Gemcitabine Plus Cisplatin Combination Therapy Followed by Radical Surgery. Ann Surg Oncol. 2015;22 Suppl 3:S1093-S1099. 11. Le VH.Outcomes of neoadjuvant therapy for cholangiocarcinoma: A review of existing evidence assessing treatment response and R0 resection rate. J Surg Oncol. 2021 Jan;123(1):164-171. 12. Aliberti C.Chemoembolization with Drug-eluting Microspheres Loaded with Doxorubicin for the Treatment of Cholangiocarcinoma. Anticancer Res.2017;37:1859-1863. 13. Schiffman SC.Precision hepatic arterial irinotecan therapy in the treatment of unresectable intrahepatic cholangiocellular carcinoma: optimal tolerance and prolonged overall survival. Ann Surg Oncol. 2011;18:431-438. 14. Kuhlmann JB.Treatment of unresectable cholangiocarcinoma:conventional transarterial chemoembolization compared with drug eluting bead-transarterial chemoembolization and systemic chemotherapy. Eur J Gastroenterol Hepatol. 2012;24:437-443. 15. Poggi G.OEM-TACE: a new therapeutic approach in unresectable intrahepatic cholangiocarcinoma. Cardiovasc Intervent Radiol. 2009;32:1187-1192. 16. Konstantinidis IT.Unresectable intrahepatic cholangiocarcinoma: Systemic plus hepatic arterial infusion chemotherapy is associated with longer survival in comparison with systemicchemotherapy alone.Cancer.2016;122:758-765. 17. Massani M, Nistri C, Ruffolo C, Bonariol R, Pauletti B, Bonariol L, Caratozzolo E, Morana G, Bassi N. Intrahepatic chemotherapy for unresectable cholangiocarcinoma: review of literature and personal experience. Updates Surg. 2015;67:389-400. 18. Le VH, O'Connor VV, Li D, Melstrom LG, Fong Y, DiFronzo AL. Outcomes of neoadjuvant therapy for cholangiocarcinoma: A review of existing evidence assessing treatment response and R0 resection rate. J Surg Oncol. 2021 Jan;123(1):164-171. 19. Riby D, Mazzotta AD, Bergeat D, Verdure L, Sulpice L, Bourien H, Lièvre A, Rolland Y, Garin E, Boudjema K, Edeline J. Downstaging with Radioembolization or Chemotherapy for Initially Unresectable Intrahepatic Cholangiocarcinoma. Ann Surg Oncol.2020. 20. Sarwar A, Ali A, Ljuboja D, Weinstein JL, Shenoy-Bhangle AS, Nasser IA, MORRow MK, Faintuch S, Curry MP, Bullock AJ, Ahmed M. Neoadjuvant Yttrium-90 Transarterial Radioembolization with Resin Microspheres Prescribed Using the Medical Internal Radiation Dose Model for Intrahepatic Cholangiocarcinoma. J Vasc Interv Radiol. 2021 Nov;32(11):1560-1568. 21. Schartz DA, Porter M, Schartz E, Kallas J, Gupta A, Butani D, Cantos A. Transarterial Yttrium-90 Radioembolization for Unresectable Intrahepatic Cholangiocarcinoma: A Systematic Review and Meta-Analysis. J Vasc Interv Radiol. 2022 Jun;33(6):679-686. 22. Gupta AN, Gordon AC, Gabr A, Kalyan A, Kircher SM, Mahalingam D, Mulcahy MF, Merkow RP, Yang AD, Bentrem DJ, Caicedo-Ramirez JC, Riaz A, Thornburg B, Desai K, Sato KT, Hohlastos ES, Kulik L, Benson AB, Salem R, Lewandowski RJ. Yttrium-90 Radioembolization of Unresectable Intrahepatic Cholangiocarcinoma: Long-Term Follow-up for a 136-Patient Cohort. Cardiovasc Intervent Radiol.2022 Aug;45(8):1117-1128. 23.Akateh C. Neoadjuvant treatment strategies for intrahepatic cholangiocarcinoma. World J Hepatol 2020; 12(10): 693-708.